Diffusion

Content:
Definition
The word “diffusion” of Latin origin – “diffusio” in Latin means “spread, dispersion.” In science, diffusion is the process of the interpenetration of microparticles upon the contact of different materials. The academic definition of diffusion is: “Diffusion is the mutual penetration of the molecules of one substance into the intermolecular spaces of another substance due to their chaotic motion and collision with each other.”
Reasons
The cause of diffusion is the thermal motion of particles (atoms, molecules, ions, etc.).
To understand in more detail how diffusion mechanisms work, we explain this phenomenon using a specific example. If we take potassium permanganate (KMnO4) and dissolve in water (H2O), then potassium permanganate will decompose into K+ and MnO4– as a result of dissociation. It is also important to note that the water molecule is polarized and exists in the form of coupled ions H + – OH-.
A chaotic movement of ions of both substances will occur due to the dissolution of potassium permanganate in water. As a result, the coupled water ions will change their color and make room for others, not yet reacted ions. Water will change its color and will get specific properties. Diffusion will occur between water and potassium permanganate.
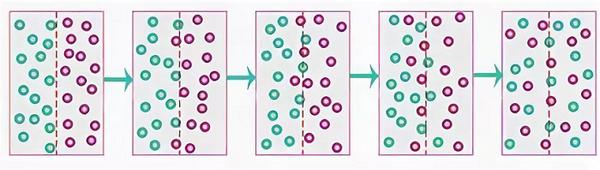
This is how this process looks schematically.
Movable particles always spread equally during diffusion throughout the entire volume. The diffusion process itself takes a certain time.
It is also important to know that the phenomenon of diffusion does not occur with all substances. For example, if the water is mixed with oil, then there will be no diffusion between them since the oil molecules are electrically neutral. The formation of a compound with water molecules is prevented by strong bonds inside the oil molecule.
The diffusion rate will increase significantly with increasing temperature, which is quite logical, because with increasing temperature the speed of movement of particles inside the substance will increase. As a result, the chance of their penetration into the molecules of another substance will increase.
Formula
The diffusion process in a two-component system is written using Fick’s law:
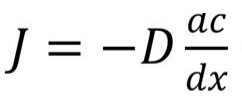
In this equation, J is the density of the material, D is the diffusion coefficient, and ac/dx is the concentration gradient of the two substances.
A diffusion coefficient is a physical quantity that is numerically equal to the amount of a diffusing substance. It is important to note that the diffusion coefficient depends on temperature.
Now let’s talk about different types of diffusion.
Examples in Solids
Diffusion in solids occurs very slowly. Solids are characterized by the presence of a crystal lattice, and all particles are arranged in an ordered manner.
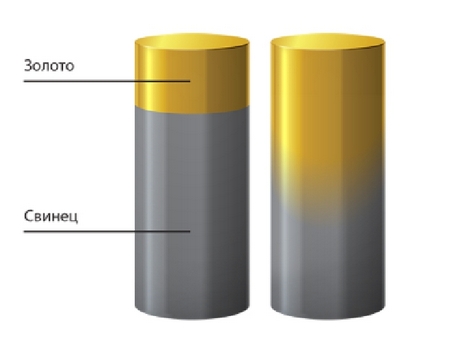
An example of diffusion of solids is gold and lead. Located at a distance of 1 meter from each other, at room temperature of 20 ° C (68 °F), these substances will gradually penetrate each other, but it will all go very slowly. Such diffusion will become noticeable no earlier than after 4-5 years.
Examples in Liquids
The diffusion in liquids is several times higher than in solids. The bonds between particles in a liquid are much weaker (usually their energy is enough for a maximum to form droplets), and nothing interferes with the mutual penetration of particles into the molecules of two substances.
However, the rate of diffusion depends on the nature and consistency of liquids. It proceeds more slowly in denser solutions because the denser the liquid, the stronger the bonds between the molecules in it and the more difficult it is for molecules and particles to penetrate each other. For example, mixing two liquid metals can take several hours, while mixing water and potassium permanganate is carried out in a minute.

Examples in Gases
The diffusion in gases occurs even faster than in liquids. There are practically no bonds between particles of gaseous substances, and unlinked particles easily mix, penetrating molecules of other gases. Minor corrections during the diffusion of gases can be made only by gravity.
Examples in Nature
It is thanks to the diffusion:
- a uniform composition of atmospheric air is maintained near the surface of our planet,
- plants are fed,
- carried out the breathing of humans and animals.
Role in Biology
The photosynthesis is happening thanks to diffusion. Thanks to the energy of sunlight water are decomposed by chlorophylls into components. The oxygen that is released in this case enters the atmosphere and is absorbed by all living organisms. So, the very process of oxygen uptake by humans and animals, and the metabolism of plants, all this is supported by diffusion, without which Life itself could not exist.
Examples in Everyday Life
What are examples of diffusion in everyday life? We can observe diffusion:
- In the garden, where the flowers exude their aroma due to diffusion (their particles are mixed with particles of ambient air).
- Tea or coffee becomes sweet due to diffusion when you add sugar into it.
- Your eyes may start to cry when cutting an onion. This also happens thanks to diffusion; onion molecules mix with air molecules and your eyes may react to it.
There are many more examples of diffusion in plants, cells, animals, and the human body.
Osmosis
Osmosis is a special example of diffusion. It is the diffusion of a substance through a semipermeable membrane from a more dilute solution to a more concentrated solution. This process is also passive since no external energy is needed.
A semipermeable membrane is a barrier that permits the passage of some substances but not others. Cell membranes are described as selectively permeable because not only do they allow the passage of water but also allow the passage of certain solutes (dissolved substances).
Some major examples of osmosis:
- Absorption of water by plant roots.
- Reabsorption of water by the proximal and distal convoluted tubules of the nephron.
- Reabsorption of tissue fluid into the venule ends of the blood capillaries.
- Absorption of water by the alimentary canal, stomach, small intestine and the colon.
References and Further Reading
- J.G. Kirkwood, R.L. Baldwin, P.J. Dunlop, L.J. Gosting, G. Kegeles (1960)Flow equations and frames of reference for isothermal diffusion in liquids. The Journal of Chemical Physics 33(5):1505–13.
- J. Philibert (2005). One and a half century of diffusion: Fick, Einstein, before and beyond. Archived 2013-12-13 at the Wayback Machine Diffusion Fundamentals, 2, 1.1–1.10.
- S.R. De Groot, P. Mazur (1962). Non-equilibrium Thermodynamics. North-Holland, Amsterdam.
- A. Einstein (1905). “Uber die von der molekularkinetischen Theorie der Warme geforderte Bewegung von in ruhenden Flussigkeiten suspendierten Teilchen” (PDF). Ann. Phys. 17 (8): 549–60. Bibcode:1905AnP…322..549E. doi:10.1002/andp.19053220806.
- Diffusion Processes, Thomas Graham Symposium, ed. J.N. Sherwood, A.V. Chadwick, W.M.Muir, F.L. Swinton, Gordon and Breach, London, 1971.

Author: Pavlo Chaika, Editor-in-Chief of the journal Poznavayka
When writing this article, I tried to make it as interesting and useful as possible. I would be grateful for any feedback and constructive criticism in the form of comments to the article. You can also write your wish/question/suggestion to my mail pavelchaika1983@gmail.com or to Facebook.

