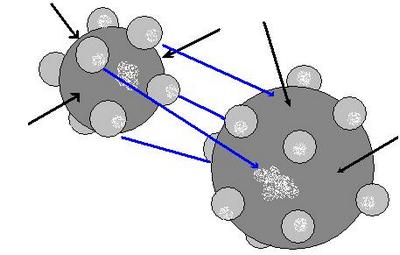
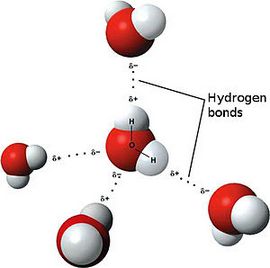
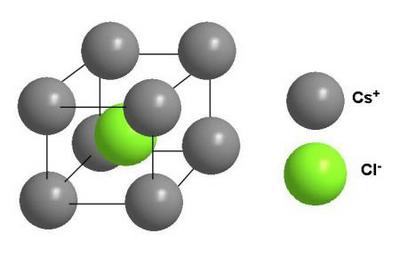
Avogadro’s Law
The Avogadro law was formulated by the Italian chemist Amadeo Avogadro in 1811. It had great importance for the development of the chemistry of that time. However, Avogadro’s law has not lost its relevance and significance even today. Let’s try to define Avogadro law; it will sound something like this.