Crystal Lattice in Chemistry
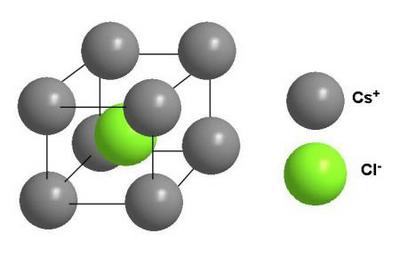
Content:
Definition
All material substances exist in three basic states: liquid, solid, and gaseous. Yet, there is a state of plasma, which scientists consider to be the fourth state of matter, but our article is not about plasma. Do you know what table salt and a beautiful diamond have in common? Based on their structure, they are both solid objects. The solid objects (whether diamond or salt) have a special crystalline structure; they contain tiny interlocking crystals. This crystalline structure has a certain order, creating a crystal lattice. The crystal lattice is the arrangement of atoms in a crystal.
Structure
The structure of a crystal lattice consists of small unit cells: atoms, molecules, ions, and other elementary particles. The structure of a crystal lattice is shown here.
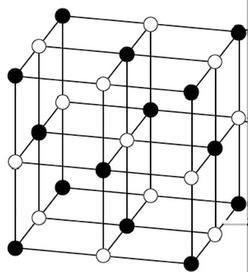
Types
There are fourteen types of crystal lattices.
The most popular among them are:
- Ionic lattice.
- Atomic lattice.
- Molecular lattice.
- Metal lattice.
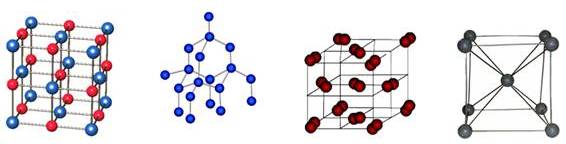
Ionic Lattice
An ionic lattice has the opposite electric charge of ions. These electric charges create an electromagnetic field, and this field determines the properties of substances having an ionic lattice: refractoriness, hardness, density and the ability to conduct electricity. Table salt is a good example of an ionic lattice.
Atomic Lattice
Substances with an atomic lattice have a strong covalent bond in their sites consisting of atoms themselves. Covalent bonds happen when two identical atoms share electrons with each other. They form a common pair of electrons for neighboring atoms. This is the reason why covalent bonds bind atoms in a strict order. Perhaps this is the most characteristic feature of the atomic lattice. Chemical elements with atomic lattices have a high melting point. Such chemical elements as diamond, silicon, germanium, and boron have atomic lattices.
Molecular Lattice
A molecular lattice is characterized by the presence of stable and closely-packed molecules. They are located in the nodes of the crystal lattice. They are held by the Van der Walsh forces that are ten times weaker than the forces of the ionic interaction. Ice is a good example of a molecular lattice – a solid substance, which has the property of passing into a liquid matter. The bonds between the lattice molecules are quite weak.
Metal Lattice
A metal lattice structure is more flexible and plastic than the ionic one, although outwardly they are very similar. Metal lattices have positively charged metal ions in the lattice sites. There are electrons between the nodes, which are involved in the creation of an electric field. Sometimes these electrons are called electric gas. The metal lattice structure explains its properties: mechanical strength, heat, electrical conductivity, and fusibility.
References and Further Reading
- Petrenko, V. F.; Whitworth, R. W. (1999). Physics of Ice. Oxford University Press. ISBN 9780198518945.
- Bernal, J. D.; Fowler, R. H. (1933). “A Theory of Water and Ionic Solution, with Particular Reference to Hydrogen and Hydroxyl Ions”. The Journal of Chemical Physics. 1 (8): 515. Bibcode:1933JChPh…1..515B. doi:10.1063/1.1749327.
- Hook, J.R.; Hall, H.E. (2010). Solid State Physics. Manchester Physics Series (2nd ed.). John Wiley & Sons. ISBN 9780471928041.
- West, Anthony R. (1999). Basic Solid State Chemistry (2nd ed.). Wiley. p. 1. ISBN 978-0-471-98756-7.
- International Tables for Crystallography (2006). Volume A, Space-group symmetry.

Author: Pavlo Chaika, Editor-in-Chief of the journal Poznavayka
When writing this article, I tried to make it as interesting and useful as possible. I would be grateful for any feedback and constructive criticism in the form of comments to the article. You can also write your wish/question/suggestion to my mail pavelchaika1983@gmail.com or to Facebook.

