Brownian Motion
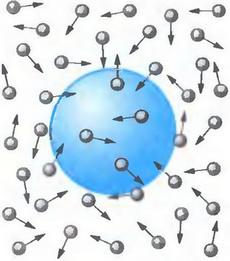
Content:
Definition
Brownian motion (or Brownian movement) is the chaotic and random motion of small particles (usually molecules) in different liquids or gases. The cause of Brownian motion is the collision of small particles with other particles. What is the story of the discovery of Brownian motion? Why is Brownian motion so important in physic and chemistry? What are some examples of Brownian motion in real life? Read about all this in our article.
Discovery
The discoverer of the Brownian movement was the English botanist Robert Brown (1773-1858). It is in his honor it was called “Brownian”. In 1827, Robert Brown was engaged in active research on pollen from various plants. He was particularly interested in how pollen takes part in plant propagation. One day, observing the movement of pollen in vegetable juice in a microscope, the scientist noticed that small particles make random tortuous movements.
This observation was confirmed by other scientists. In particular, it was noted that particles tend to accelerate with increasing temperature, as well as with decreasing particle size.

Robert Brown, discoverer of the Brownian movement.
At first, Robert Brown thought that he was observing the movement, even the “dance” of some living microorganisms, because the pollen itself is, in fact, the male reproductive cells of plants. But particles of dead plants (and even plants dried up a hundred years ago in herbaria) had a similar movement. The scientist was even more surprised when he began to study inanimate matter: small particles of coal, soot, and even dust particles of London air. Then glass, various and various minerals came under the microscope of the researcher. These “active molecules” were observed everywhere, being in constant and chaotic motion.
This is interesting: you can observe the Brownian motion with your own eyes, for this you’ll need a strong microscope (after all, there were no powerful modern microscopes during the life of Robert Brown). If you’ll look through this microscope on a blackened box with smoke and illuminated by a wide beam of light, you’ll see small pieces of soot and ash that will continuously jump back and forth. This is a Brownian motion.
Atomic-molecular Theory
Brownian Motion soon became very famous in scientific circles. Robert Brown was happy to show it to many of his colleagues. However, scientists could not explain the reasons for the Brownian movement for many years. Moreover, the Brownian movement was completely random and did not succumb to any logic.
Its explanation was given only at the end of the 19 century, and it was not immediately accepted by the scientific community. In 1863, the German mathematician Ludwig Christian Wiener suggested that Brownian motion happens due to the vibrational movements of certain invisible atoms. This was the first explanation of this strange phenomenon, associated with the properties of atoms and molecules, the first attempt to reveal the secret of the structure of matter using Brownian motion.
Later, Wiener’s ideas were developed by other scientists. The famous Scottish physicist and chemist William Ramsay was among them. He managed to prove that the cause of the Brownian motion of small particles is the impact of even smaller particles, which are no longer visible in a normal microscope. Just as the waves swinging a distant boat are not visible from the shore, although the movement of the boat itself is visible.

William Ramsay in his laboratory.
Brownian motion became one of the important components of atomic-molecular theory and at the same time an important proof of the fact that all matter consists of the smallest particles: atoms and molecules. This is hard to believe, but some scientists denied the atomic-molecular theory and did not believe in the existence of molecules and atoms even at the beginning of the 20 century. Ramsay’s scientific works related to the Brownian movement dealt a crushing blow to the opponents of atomism and made all scientists finally convinced that atoms and molecules exist.
Theory
Despite the chaotic motion of particles, scientists still tried to describe their random movements by mathematical formulas. The theory of Brownian motion was born.
By the way, one of those who developed this theory was the Polish physicist and mathematician Marian Smolukhovsky, who at that time worked at the University of Lviv and lived in the hometown of the author of this article, in the beautiful Ukrainian city of Lviv.
Besides Marian Smolukhovsky, the famous Albert Einstein also worked on this problem.

As a result, both scientists created their theory, which can also be called the Smoluchowski-Einstein theory. In particular, a mathematical formula was formed, according to it, the mean square of the displacement of a Brownian particle ( s 2 ) over time t is directly proportional to the temperature T and inversely proportional to the viscosity of the liquid n, the particle size r, and constant Avogadro.
NA: s2 = 2RTt/6phrNA – this is what this formula looks like.
R in the formula is the gas constant. So, if in 1 minute a particle with a diameter of 1 microns moves by 10 microns, then in 9 minutes – by 10 = 30 microns, in 25 minutes – by 10 = 50 microns, etc. Under similar conditions, a particle with a diameter of 0.25 microns for the same time periods (1, 9 and 25 min) will shift by 20, 60 and 100 microns, respectively, since = 2. It is important that the Avogadro constant is included in the above formula. Avogadro constant can be determined by quantitative measurements of the motion of a Brownian particle, which was done by the French physicist Jean Baptiste Perrin.
To observe Brownian particles, Perrin used the latest ultramicroscope at that time, through which the smallest particles of matter were already visible. The scientist noted the positions of certain Brownian particles at regular time intervals (for example, after 30 seconds). Then, combining the positions of the particles with straight lines, he obtained a variety of intricate trajectories of their motion. All this was painted on a special, fragmented sheet.
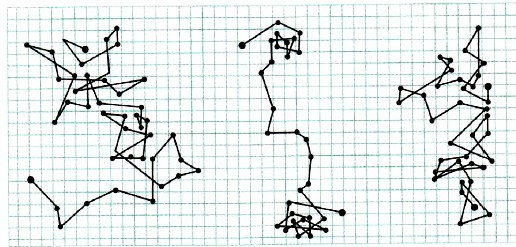
So these drawings looked.
Composing Einstein’s theoretical formula with his observations, Perrin was able to obtain the most accurate Avogadro number for that time: 6,8.1023
With his experiments, he confirmed the theoretical conclusions of Einstein and Smolukhovsky.
Diffusion
The movement of particles during Brownian motion is very similar to the movement of particles during diffusion – the mutual penetration of molecules of different substances under the influence of temperature. What is the difference between Brownian motion and diffusion? In fact, both diffusion and Brownian motion occur due to the chaotic thermal motion of molecules, and as a result are described by similar mathematical rules.
The difference between them is that during diffusion, the molecule always moves in a straight line until it collides with another molecule, after which it will change the trajectory of its movement. The Brownian particle does not make “free flight”, but experiences very small and frequent “tremors”, as a result of which it randomly moves here and there. In figurative language, the Brownian particle is like an empty can of beer lying on the square, where a large crowd of people gathered. People go back and forth, touch the can with their legs and it flies randomly in different directions like a Brownian particle. The movement of people in the crowd is already more characteristic for the movement of particles during diffusion.
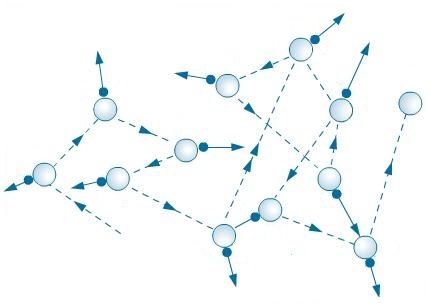
If we look at the micro-level, then the cause of the motion of the Brownian particle is its collision with smaller particles, while during diffusion the particles collide with similar other particles.
Both diffusion and Brownian motion occur under the influence of temperature. With decreasing temperature, the Brownian particle and the particle during diffusion slow down.
Examples
What are examples of Brownian motion in everyday life? The theory of Brownian motion has a practical embodiment in real life. For example, why, does a man who gets lost in the forest periodically return to the same place? Because he does not walk in circles, but approximately in the same way as a Brownian particle usually moves. Therefore, he crosses his path many times.
Without clear guidelines and directions of movement, a lost man is like a Brownian particle performing chaotic movements. To get out of the forest, you need to have clear guidelines, develop a system, instead of committing various meaningless actions. You should not behave like a Brownian particle in life, rushing from side to side, but know your direction, goal, and calling, have dreams, courage, and perseverance to achieve them.. So from physics, we smoothly moved on to philosophy.
References and Further Reading
- Teaching the Growth, Ripening, and Agglomeration of Nanostructures in Computer Experiments, Jan Philipp Meyburg and Detlef Diesing, Journal of Chemical Education, (2017), 94, 9, 1225-1231, J.Chem.Edu
- Jump up to: a b Feynman, R. (1964). “The Brownian Movement”. The Feynman Lectures of Physics, Volume I. pp. 41-, 1.
- Jump up to: a b Einstein, Albert (1905). “Uber die von der molekularkinetischen Theorie der Warme geforderte Bewegung von in ruhenden Flussigkeiten suspendierten Teilchen” [On the Movement of Small Particles Suspended in Stationary Liquids Required by the Molecular-Kinetic Theory of Heat] (PDF). Annalen der Physik (in German). 322 (8): 549–560. Bibcode:1905AnP…322..549E. doi:10.1002/andp.19053220806.
- “The Nobel Prize in Physics 1926”. NobelPrize.org. Retrieved 29 May 2019.
- Perrin, Jean (1914). Atoms. London : Constable. p. 115.

Author: Pavlo Chaika, Editor-in-Chief of the journal Poznavayka
When writing this article, I tried to make it as interesting and useful as possible. I would be grateful for any feedback and constructive criticism in the form of comments to the article. You can also write your wish/question/suggestion to my mail pavelchaika1983@gmail.com or to Facebook.


i really liked your writing. :) Thank you.